We’re here to provide you with more information or help answer any questions you might have. Send us a note and we’ll get back to you as soon as possible.


You asked, we answered! Our global Solution Centers’ pharmaceutical experts have responded to your most frequently asked questions about how our formulation lab services can help you select the right ingredients to build an optimal medication.
When we hear about pharmaceutical innovation, we only hear about famous accidents throughout history. Among the most well-known successes include John Cade’s lithium discovery for sedation, warfarin for blood thinners (originating from cows eating spoiled clover), and – perhaps most famously – penicillin's fortunate beginning from an open petri dish found after Andrew Fleming’s vacation.
While those stories make it seem like the most significant pharmaceutical breakthroughs frequently happen by accident, that’s far from the truth. Innovation in the pharma industry innovation is rarely happenstance. Instead, it’s a deliberate trial and error process with meticulous research throughout all stages of product development.
Our team of chemists, compounders, and application development specialists in our pharmaceutical labs can attest to this from their own experiences working directly with our customers and suppliers to solve formulation and drug development challenges.
How our labs help: Pharmaceutical ingredient research and development
What is pharmaceutical research and development exactly? Pharmaceutical R&D refers specifically to pharma research and development surrounding new medications before going to market.
Through Solution Centers around the world, Univar Solutions has global pharma laboratories that can partner with you through the stages of product development and commercialization. From comparative testing of excipients to compatibility analysis of APIs in formulations, these specialty labs focus on quality assurance, stability testing, and meeting regulatory requirements through a broad range of services specifically designed for the pharmaceutical industry.

8 FAQs about how our pharma labs in North America, EMEA, and Latin America support pharma
Our lab experts, including Jessica Zhang, manager of pharma technical services for North America, Eduardo Adachi, pharma laboratory analyst for Brazil, and Arianne Matos, pharma business development manager for Latin America have compiled a list to answer the most frequently asked questions about how our pharmaceutical lab services in North America, EMEA, and Latin America support pharmaceutical solutions for suppliers, formulators, and customers.
1. Where are Univar Solutions pharma labs located?
Across the globe! You can collaborate with pharmaceutical formulation research and development within our Solution Centers in Houston, Mexico City, São Paulo, and Essen. Our expert lab teams work across borders and disciplines sharing findings and discoveries to drive innovative formulation solutions for our customers and suppliers worldwide – at any stage of the product development process.
2. How do these specialty formulation labs support new product development?
It's never too early (or too late) to get started. Univar Solutions can help support you from product conception to working prototypes, including services such as:
- Formulation development according to needs
- Raw material sourcing and recommendations
- Stability testing
- Deformulation
- Specification development
- Solutions for troubleshooting new or existing formulations
- Technical support for our customers and suppliers
Don't have lab space or the personnel for your project? Univar Solutions pharma labs provide resources such as time, facility space, and additional expertise to get your latest innovations and reformulations on the production line.
3. What are some of the dosage forms that the labs support?
Our Solution Centers’ pharma labs use an expansive global reach to cover a wide range of dosage forms for your products, including tablets, capsules, coated tablets, semi-solids, liquids, and nutraceuticals. Additionally, our specialists can work with non-hazardous and non-controlled active pharmaceutical ingredients (APIs) during the development process at any stage.
4. What equipment types, batch sizes, and capabilities do Univar Solutions pharma labs offer?
Our approach is the versatility to work with all customers – whether scaling large or small projects. Our North American lab offers various mixing and filling equipment to support the dosage forms noted above, with benchtop capabilities of up to 4L for semi-solids and liquids and up to 700g for solids. Our Latin American pharma lab capabilities include 3kg for tablets, 500g for coated tablets, and up to 10L for liquids/semi-solids.
5. Are the pharma labs in your global Solution Centers FDA-approved and/or cGMP?
While they are not, it is essential to understand that our Solution Centers focus on the upstream portion of the product life cycle. We help you bring your products to market faster through various means of initial formulation and specification development, prototype and sample development, and stability testing.
6. What is analytical testing, and what types do the Univar Solutions pharma labs offer?
Analytical testing – also called materials testing – describes a battery of testing techniques used to identify a particular sample's characteristics, chemical characterization, and chemical makeup through failure analysis or reverse engineering, among other methods.
Our pharma labs support an array of chemical analysis, product performance analysis, and numerous other types of invaluable testing services.
The North American pharma lab at the Houston Solution Center has significant analytical capabilities including stability testing, various forms of chromatography (HPLC, LC-MS, GC), water content, particle size, microbiology/challenge testing, and spectroscopy (FTIR, UV-VIS).
Analytical testing at our São Paulo Solution Center includes microbiology capabilities (USP preservatives challenge test), QC tests, water content, spectroscopy UV (with specified standards), PH, and viscosity testing.
7. What are some examples of how you improved pharmaceutical R&D productivity for a customer?
Reliability is a tremendous factor that pushes innovation forward. When customer timetables have faced delays, they brought in our lab solutions expertise for faster go-to-market capabilities, which helped them strategize to get back on track. Most notably, our labs use in-house testing and technical experts rather than outsourcing the majority of R&D.
8. Do you have any examples of your stability testing in pharmaceutical products?
Our in-house analytical capabilities allow us to put products through stability tests – specifically in stability chambers – then perform accelerated testing.
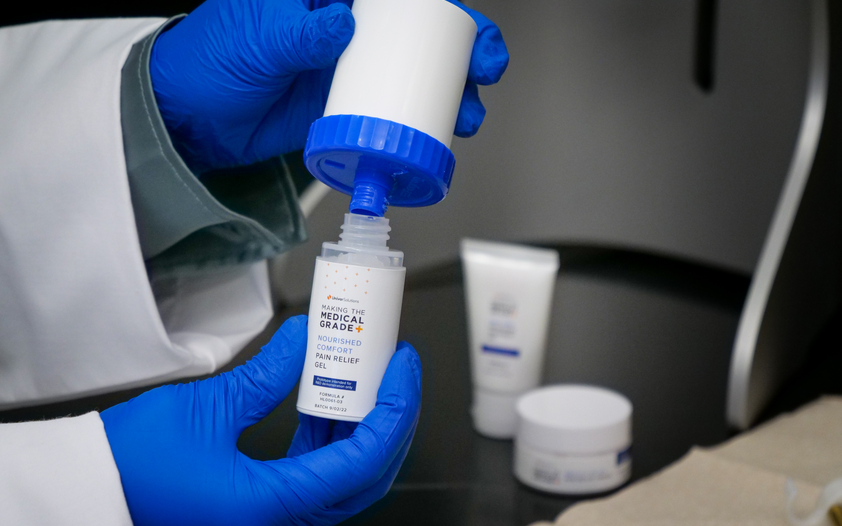
For example, when formulating our Making the Medical Grade topical kit, our specialists identified high-quality, high-purity ingredients from leading producers to develop three unique topical treatment formulas. These prototypes offer standout ingredients and techniques that underwent accelerated stability testing. The excipients and ingredients were verified to enhance product lines by offering more effectiveness and appeal to physicians and consumers.
Not every breakthrough can be left to chance. Have great pharma discoveries occurred by accident? Absolutely. But our collaborators know firsthand from partnerships with our chemists, researchers, and technical specialist teams that medicinal innovation is usually a methodical process with no margin for accidents when getting your products to market sooner.
Formulate and innovate with the latest pharmaceutical ingredients. Collaborate with our labs to find optimal formulation solutions led by the top pharma ingredient technical experts.
1. Where are Univar Solutions pharma labs located?
Across the globe! You can collaborate with pharmaceutical formulation research and development within our Solution Centers in Houston, Mexico City, São Paulo, and Essen. Our expert lab teams work across borders and disciplines sharing findings and discoveries to drive innovative formulation solutions for our customers and suppliers worldwide – at any stage of the product development process.
2. How do these specialty formulation labs support new product development?
It's never too early (or too late) to get started. Univar Solutions can help support you from product conception to working prototypes, including services such as:
- Formulation development according to needs
- Raw material sourcing and recommendations
- Stability testing
- Deformulation
- Specification development
- Solutions for troubleshooting new or existing formulations
- Technical support for our customers and suppliers
Don't have lab space or the personnel for your project? Univar Solutions pharma labs provide resources such as time, facility space, and additional expertise to get your latest innovations and reformulations on the production line.
3. What are some of the dosage forms that the labs support?
Our Solution Centers’ pharma labs use an expansive global reach to cover a wide range of dosage forms for your products, including tablets, capsules, coated tablets, semi-solids, liquids, and nutraceuticals. Additionally, our specialists can work with non-hazardous and non-controlled active pharmaceutical ingredients (APIs) during the development process at any stage.
4. What equipment types, batch sizes, and capabilities do Univar Solutions pharma labs offer?
Our approach is the versatility to work with all customers – whether scaling large or small projects. Our North American lab offers various mixing and filling equipment to support the dosage forms noted above, with benchtop capabilities of up to 4L for semi-solids and liquids and up to 700g for solids. Our Latin American pharma lab capabilities include 3kg for tablets, 500g for coated tablets, and up to 10L for liquids/semi-solids.
5. Are the pharma labs in your global Solution Centers FDA-approved and/or cGMP?
While they are not, it is essential to understand that our Solution Centers focus on the upstream portion of the product life cycle. We help you bring your products to market faster through various means of initial formulation and specification development, prototype and sample development, and stability testing.
6. What is analytical testing, and what types do the Univar Solutions pharma labs offer?
Analytical testing – also called materials testing – describes a battery of testing techniques used to identify a particular sample's characteristics, chemical characterization, and chemical makeup through failure analysis or reverse engineering, among other methods.
Our pharma labs support an array of chemical analysis, product performance analysis, and numerous other types of invaluable testing services.
The North American pharma lab at the Houston Solution Center has significant analytical capabilities including stability testing, various forms of chromatography (HPLC, LC-MS, GC), water content, particle size, microbiology/challenge testing, and spectroscopy (FTIR, UV-VIS).
Analytical testing at our São Paulo Solution Center includes microbiology capabilities (USP preservatives challenge test), QC tests, water content, spectroscopy UV (with specified standards), PH, and viscosity testing.
7. What are some examples of how you improved pharmaceutical R&D productivity for a customer?
Reliability is a tremendous factor that pushes innovation forward. When customer timetables have faced delays, they brought in our lab solutions expertise for faster go-to-market capabilities, which helped them strategize to get back on track. Most notably, our labs use in-house testing and technical experts rather than outsourcing the majority of R&D.
8. Do you have any examples of your stability testing in pharmaceutical products?
Our in-house analytical capabilities allow us to put products through stability tests – specifically in stability chambers – then perform accelerated testing.
For example, when formulating our Making the Medical Grade topical kit, our specialists identified high-quality, high-purity ingredients from leading producers to develop three unique topical treatment formulas. These prototypes offer standout ingredients and techniques that underwent accelerated stability testing. The excipients and ingredients were verified to enhance product lines by offering more effectiveness and appeal to physicians and consumers.
Not every breakthrough can be left to chance. Have great pharma discoveries occurred by accident? Absolutely. But our collaborators know firsthand from partnerships with our chemists, researchers, and technical specialist teams that medicinal innovation is usually a methodical process with no margin for accidents when getting your products to market sooner.
Formulate and innovate with the latest pharmaceutical ingredients. Collaborate with our labs to find optimal formulation solutions led by the top pharma ingredient technical experts.